Structural remodeling of ribosome associated Hsp40-Hsp70 chaperones during co-translational folding
2022-06-15
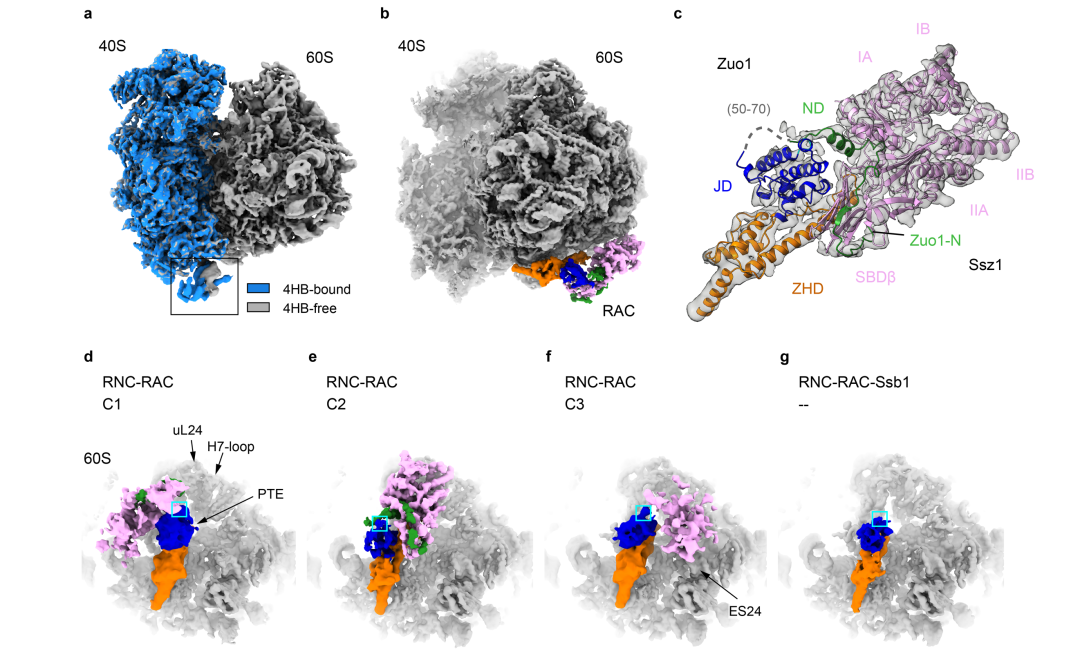
Ribosome associated complex(RAC), an obligate heterodimer of HSP40 and HSP70(Zuo1and Ssz1 in veast is conserved in eukarvotes and functions as co-chaperone for another HSP70(Sso1/2 in yeast)to facilitate co-translational folding of nascent polypeptides.Many mechanistic details, such as the coordination of one HSP40 with two HSP70s and the dynamic interplay between RAC-Ssb and growing nascent chainsremain unclear.Herewe report three sets of structures of RAC-containing ribosomal complexes isolated from Sac charomvces cerevisiae.Structural analvses indicate that RAC on the nascent-chain-free ribo-some is in an autoinhibited conformation, and in the presence of a nascent chain at the peptide tunnel exit(PTE)RAC undergoes large-scale structural remodeling to make Zuo1 J-Domain more accessible to Ssb.Our data also suggest a role of Zuo1 in orienting Ssb-SBD proximal to the PTE for easy capture of the substrate.Altogetherin accordance with previous data, our work suggests a sequence of structural remodeling events for RAC-Ssb during co- translational folding, triggered by the binding and passage of growing nascent chain from one to another.